Chemical Reactions That Changed World History
Chemical reactions are constantly occurring all around us, even within our own bodies. Over millennia, humans have learned to exploit some of these chemical reactions to their advantage.

From the discovery of soap to the creation of bronze tools, this 'modern magic' has completely changed entire civilizations. If you want to learn more about the impact of chemistry on the world, read on to discover how these chemical reactions have changed the course of history!
Saponification

Around 2,800 BC, humans discovered that by mixing just three simple ingredients—animal fat, wood ash, and water—we could create soap. Although simple, it is a chemical reaction and its name is saponification.
At first, we mainly used soap to clean other materials, such as cotton and wool. However, over time, people realized that soap could also clean the body.
When microorganisms were discovered, soap became even more important as a cleanser, especially when we realized that it could prevent disease.
Although the soap making process has undergone many changes, at its core it is still the same simple reaction we have been performing for thousands of years.
Synthetic ammonia

Fritz Haber revolutionized agriculture in 1909 by developing a process to extract nitrogen from the air and convert it into ammonia, a usable form of nitrogen. His colleague Carl Bosch later scaled up the process, creating nitrogen fertilizer. But what makes this chemical reaction so important?
Plants need nitrogen to grow, which they usually get from the soil. However, after many harvest cycles, crop yields decrease as nitrogen levels drop. Nitrogen makes up about 78% of our atmosphere, but plants cannot use it in this form. Some microorganisms can fix nitrogen into the soil, but this is a slow process.
Traditionally, different crops were grown in rotation in fields to maintain balanced nutrition. With the Haber-Bosch process, famine was prevented and we moved one step closer to industrial agriculture.
Then World War I happened. The Nazis had plenty of ammonia to make more ammunition, and Haber went on to invent chlorine gas.
Maillard reaction

You may have never heard of the Maillard reaction, but in fact, you're using it all the time in your kitchen.
It's the secret behind the crispy brown crust on fresh bread and the delicious crust on a seared steak; it's the hero in disguise behind crème brûlée.
So what happens during the Maillard reaction? During cooking, the sugars and proteins in food undergo a chemical reaction from the heat, turning them into thousands of compounds that give food its flavor and aroma.
Ancient humans discovered that cooked food smelled and tasted better, was easier to digest, and kept them fuller longer.
Discovery of penicillin
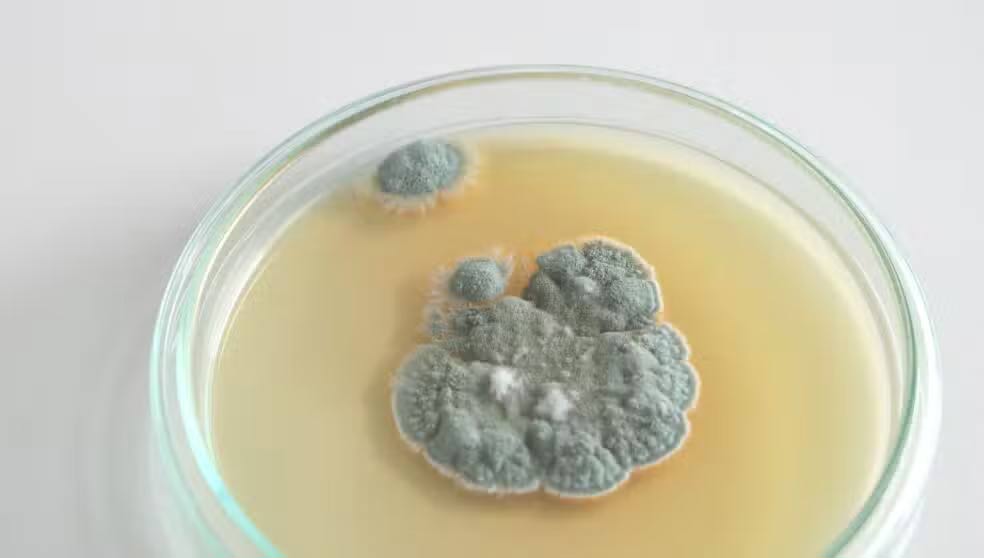
In 1928, Dr. Alexander Fleming walked into his laboratory after a vacation and discovered something unusual: A fungus had contaminated the petri dish he was using to grow Staphylococcus bacteria.
Rather than throw away the contaminated sample, he decided to take a closer look. That unusual decision led to a surprising breakthrough. He noticed that bacteria did not grow near fungi, so he investigated further.
This mold, later identified as Penicillium notatum, produced a substance that killed bacteria by breaking down their cell walls.
Despite Fleming's best efforts, he was unable to isolate the active ingredient produced by the fungus. More than a decade later, Dr. Howard Florey, Dr. Ernst Chain, and a team of other scientists at Oxford University succeeded in producing a crude extract from the mold.
Far from perfect, the original process required 528 gallons (2,000 liters) of mold culture to produce enough penicillin for a critically ill person. By the 1940s, improved processes were being used to grow crops, and penicillin ushered in the antibiotic era, saving countless lives.
Chlorofluorocarbon synthesis

Chlorofluorocarbons, also known as Freons or CFCs, were invented by Thomas Midgley Jr. in the 1920s. At that time, refrigerators used extremely toxic refrigerants such as ammonia, methyl chloride, and sulfur dioxide.
CFCs, a non-toxic, non-flammable, and stable chemical, seemed like the perfect alternative. Unfortunately, no one knew that CFCs break down under UV light. By the 1950s, CFCs were everywhere, from refrigerators and air conditioners to aerosol cans. CFCs eventually drifted into the upper atmosphere, where they reacted with UV light from the Sun and released chlorine.
Chlorine gradually ate away at the ozone layer, allowing more of the sun's harmful rays to penetrate. It wasn't until 1974 that scientists predicted the damage, and by then it was almost too late.
The Montreal Protocol was eventually signed by most of the world's nations in 1987 and, with some necessary exceptions, resulted in an effective ban on all CFC production worldwide.
Somehow, it worked: Our planet is slowly recovering its ozone layer. For the first time, all UN members unanimously adopted a treaty, making the CFC phaseout a landmark moment in UN history.
You should read it
- ★ Amazing discovery, chemical elements do not follow quantum mechanics theory
- ★ Please refer to the useful application for learning, with download link
- ★ Is it possible that someday cells in your body can connect to your smartphone?
- ★ How to type chemical formulas in Word
- ★ Guide interactive statistics Reactions when Livestream on Facebook