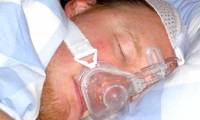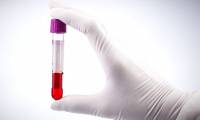
The US FDA allows transfusion of cured human plasma to treat serious cases of Covid-19 but is prohibited for use in healthy individuals
the fda has temporarily granted authorization for this treatment through its waivers when applying for new drug testing (einds). in the einds, the fda says drugs that are in the