Constructing the electronic structure of benzene, a compound with electrons that exists in 126 different directions
In 1825, Michael Faraday discovered the existence of benzene when condensation of glowing gases. For a long time, we have understood the atomic structure of benzene, but since the 1930s, science has been arguing about the electronic structure of benzene. If you don't already know, in quantum chemistry, 'electronic structure' is the movement state of electrons in an electrostatic field created by many fixed nuclei.
Discovering the electronic structure of benzene, we will have the building blocks of the future of the development of optoelectronic materials.
The atomic structure of benzene is as follows: is a ring of 6 carbon atoms and 6 hydrogen atoms, linked together in pairs. But the fact that benzene has 42 electrons makes it difficult to study its electronic structure.
' The benzene function describing the electron shows that it has up to 126 dimensions, ' said chemist Timothy Schmidt. 'This means that this function has up to 126 coordinate values, 3 for each of the 42 electrons. The electrons do not work independently, so it can't be split into 42 three-dimensional functions .'
According to researcher Schmidt, the answer of computers is not easy to understand, so scientists have to find their own way to get the answer.
They had to use math to describe the electronic structure of benzene, and the formula needed to calculate all 126 dimensions to be calculated. It sounds complicated, and the nature of the problem is really tough; that's why science has been fighting for decades. Some even question whether the electrons in benzene really work to solve this difficult problem.
There are two ways of thinking: one side thinks that benzene works based on valence theory , with the normally operating electrons (localized electrons, operating in a certain range); the other party argued that benzene followed the satellite molecular theory , the electrons were indeterminate, beyond the framework and reached out to nearby atoms.
The conundrum is that both theories do not explain the electronic structure of benzene.
So a new explanation is needed, and the 'Voronoi Metropolis dynamic sampling' technique has given researchers a way out. By algorithms that describe the wave function of a multi-electron system, they solved a difficult problem.
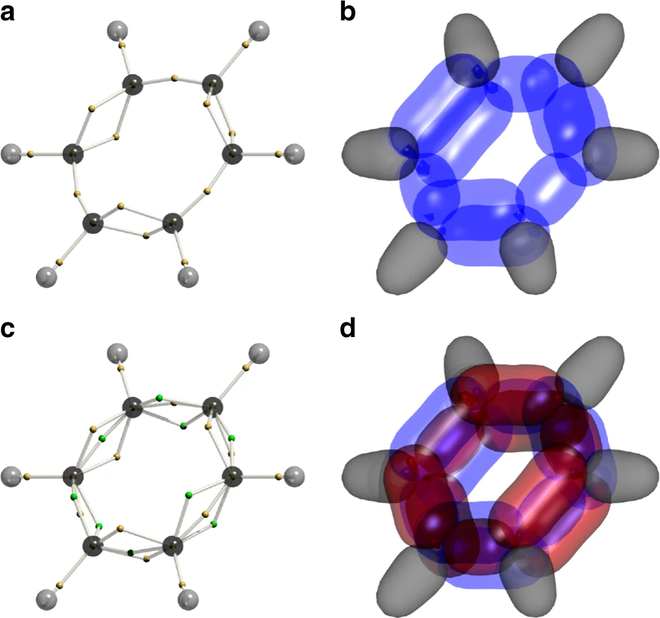
The new algorithm splits the electron directions into Voronoi diagrams, with each frame showing one electron coordinates, allowing the research team to draw the wave function diagrams of the entire 126 dimensions. And then they discovered something strange, changing the way science thinks about benzene.
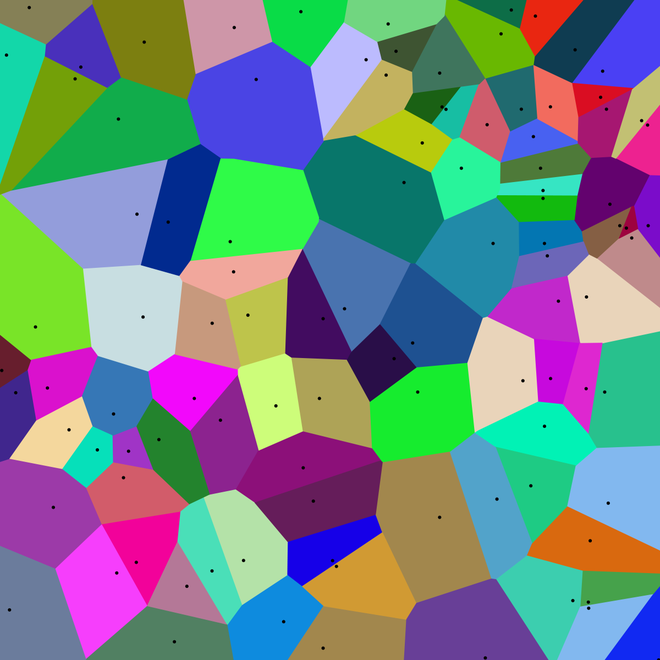
Example of Vonoroi diagram.
Research shows that electrons will 'avoid' each other when they have a chance, reducing the energy inside the molecule, thereby making the benzene structure more stable than before.
' Basically, this discovery links previous observations of the chemical properties of benzene, showing us that the two available models describing benzene can fit together ,' Professor Schmidt said. to speak.
' And now we also know how to observe electron correlation - that is, how the electrons avoid each other. This has always been overlooked, when previous calculations were only applied when the energy was changing, not taking into account the internal electrical activity . '
The new research is published in Nature.
Refer to ScienceAlert