First success in restoring vision using stem cell treatment
Three people with severe vision loss who received stem cell transplants reported significant improvements in their vision that lasted for more than a year. A fourth person with severe vision loss also had improvements, but they did not last. The four people were the first volunteers in a stem cell transplant trial to treat damaged corneas, the clear outer surface of the eye. That is the first successful trial of a stem cell treatment to restore vision, published in The Lancet today.
Dr Kapil Bharti, a translational stem cell researcher at the US National Eye Institute, said the results were impressive, 'an exciting development' and had the potential for widespread application.
Reprogrammed cells
Anatomically, the outer layer of the cornea is maintained by a reservoir of stem cells located in the limbus—the dark ring around the iris. When this vital source of rejuvenation is depleted—a condition known as limbal stem cell deficiency (LSCD)—scar tissue covers the cornea, eventually leading to blindness. Such a condition can result from eye trauma or from autoimmune and genetic diseases.
Treatment options for LSCD are limited, and almost exclusively involve transplanting corneal cells derived from stem cells taken from a healthy eyeball. This is an invasive procedure with uncertain outcomes. When both eyes are affected, corneal transplants from deceased donors are an option, but they are sometimes rejected by the recipient's immune system.
Ophthalmologist Kohji Nishida and his colleagues at Osaka University in Japan have come up with an alternative cell source — induced pluripotent stem (iPS) cells — to perform corneal transplants. They take blood cells from a healthy donor and reprogram them into an embryonic-like state, then transform them into a thin, transparent, pebble-like layer of corneal epithelial cells.
Between June 2019 and November 2020, Nishida's team enrolled two women and two men between the ages of 39 and 72 with LSCD in both eyes. As part of the surgery, the team shaved off the scar tissue covering the damaged cornea in only one eye, then sewed on epithelial sheets taken from a donor and placed a soft protective contact lens over it.
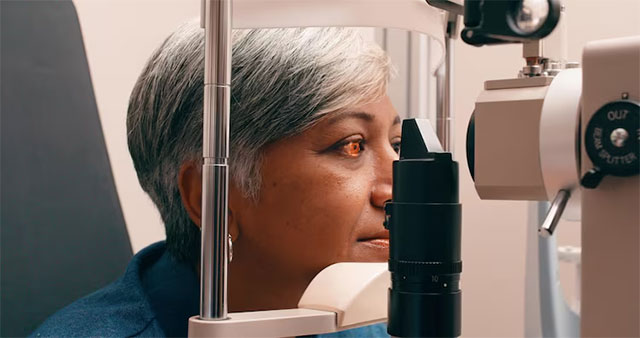
Vision test
Two years after the transplants, none of the patients experienced serious side effects. The grafts did not form tumors—a known risk of developing iPS cells—and there were no obvious signs of being attacked by the recipients' immune systems, even in the two patients who were not taking immunosuppressant and anti-rejection drugs. Of course, more cases like this will need to be done to be sure of the intervention's safety.
After stem cell transplantation, all four volunteers experienced immediate improvement in vision and a reduction in the area of the cornea affected by LSCD. The improvement continued in all but one, who showed slight reversal at one-year follow-up.
It is still unclear exactly what caused the improvement in vision. It is possible that the transplanted cells grew naturally in the recipient's cornea. But the improvement in vision could also be due to scar tissue being removed before the transplant, or the transplant triggering the recipient's own cells to migrate from other areas of the eye and rejuvenate the cornea.
The team said they plan to launch a clinical trial in March 2025 to assess the effectiveness of the new treatment. Several other iPS cell-based trials are underway globally to treat eye diseases. These early successes show that scientists are on the right track.
You should read it
- ★ What you need to know about app development for Apple Vision Pro
- ★ How is Computer Vision used to detect phishing attacks?
- ★ Strangely, people with 45% burns are healed quickly with stem cell therapy
- ★ What is the difference between Apple Vision Pro and Meta Quest 3? Which one should I buy?
- ★ Apple shows how it's possible to try out the new iPhone inside the Vision Pro