Floating equipment can obtain hydrogen gas from seawater
Hydrogen is a clean fuel, but current production methods, usually by converting natural gas, can be harmful to the environment.
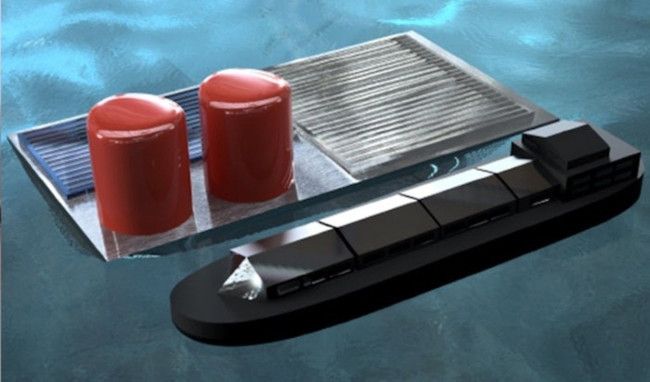
Producing hydrogen from the sun and water does not produce CO2, and recent studies have improved efficiency and lowered the cost of devices that achieve this process. Recently, Columbia University engineers are developing a "solar fuel rig" that floats on the sea, can collect energy through a solar battery and use it to collect hydrogen from seawater. .
The rig produces hydrogen through electrolysis of water, separating H2 and O2 gas from the water by passing an electric current through the liquid. Most of the time these same devices require a membrane to separate the two electrodes, but these membranes are quite fragile and need water to be very pure, this is their weakness.
Equipment developed in Columbia can divide water into hydrogen and oxygen without membranes. That means it can be deployed on seawater - the environment is easy to degrade membranes because of impurities and microorganisms.
Jack Davis, the first author of the article describing the device, said: "It is possible to safely demonstrate a device that can perform electrolysis without membranes, helping to get closer to water electrolysis These solar generators are mainly artificial photosynthesis systems, like the photosynthesis process of green plants, so our equipment can open a lot of opportunities to create energy. renewable, green and clean " .
(Photo: Daniel Esposito / Columbia Engineer)
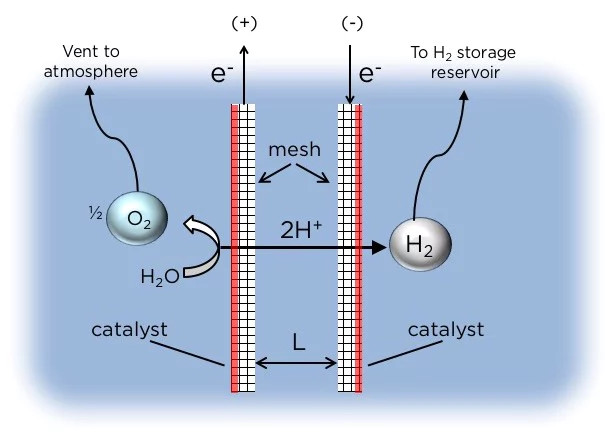
A chart shows how the research team's electrolytic system works: red lines indicate that the catalyst is mounted on one side of each electrode, H2 gas bubbles form along the lateral surface. must be collected into the collection chamber, while O2 bubbles will escape from another vent.
Instead of using membranes, the Columbia system uses two grid electrodes, which are asymmetrically designed. Each pole is coated with a catalyst only on the outside, and air bubbles will be on these surfaces. H2 bubbles formed on one electrode and O2 on the other, and to obtain these gases, this device uses simple physics - in particular, they wait for the bubble to grow large enough to emerge. surface. O2 gas that floats to the surface will escape into the air, while floating H2 (Hydro) bubbles are collected into a collection chamber.
This unique electrolytic mechanism is connected to a photovoltaic cell, producing the required current with the energy obtained from sunlight on an open sensor platform. The whole system can be mounted on a floating surface.
The study is published in the International Hydrogen Energy Journal.
See more:
- Bioplastic biodegradable in natural environment made from shrimp shells
- New molecular printing technology can reproduce a complex chemical environment similar to the human body
- Those who adapt to all habitats are the last survivors
You should read it
- ★ Without electrolysis, splashing a microwave into the water is also capable of generating hydrogen
- ★ Why is seawater salty?
- ★ Roll the list of the best family water filters on the market today
- ★ Ships traveling around the world for 6 years do not need fuel
- ★ What is hydrogen water purifier? Is hydrogen water purifier good?