Not graphene, borophene is the magic material of the future, the new darling of science
Graphene is an extremely strong carbon sheet, thickness by atomic diameter, capable of 'transforming' into different shapes and can conduct electricity. These capabilities make graphene honored by the world's scientific community as the marvelous material of the future, which can open a new era for computer microprocessors. In order to stimulate the development of the graphene industry, the EU alliance has spent almost 1 billion euros.
Although the graphene dream has not yet come true, it also helps scientists find new materials. The most prominent is borophene, a thin layer of boron atom can be classified into many different crystal structures, can be applied to many industries, used for many different purposes.

A block of borophene.
Electrochemists say that using borophene in the production of anode can create a new generation lithium-ion battery. Meanwhile, experimental physicists use borophene to search for many types of atoms and molecules.
Since the 1990s, physicists have predicted the existence of borophene through a program that simulates how boron atoms merge into a thin layer of boron.
By 2015, by depriving hot gas from concentrated boron molecules to a cold surface made of pure steel, the scientists synthesized borophene.
Boron atoms are squeezed according to the arrangement of iron atoms, with each of the six atoms joined together into a hexagon. However, a large proportion of boron atoms have a different, more specific structure, with only 4 or 5 atoms joining together to form holes in the hexagonal structure. That same hole makes the borophene special.
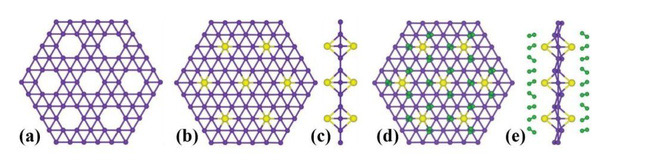
Borophene is an artificial product, so scientists have conducted many different tests to give it more unique properties. From those tests, scientists have discovered that borophene is even more solid and flexible than graphene. In addition, borophene also has superconducting properties, which can transmit electricity and heat very well.
It is the gaps in the borophene structure that create these properties. Changing gaps is to change properties.
In addition to these properties, borophene is very light and reacts well to many substances. Therefore, scientists consider borophene to be a candidate for ion storage in batteries.
Borophene can also become an effective hydrogen storage tool because hydrogen atoms can easily adhere to the borophene structure. Hypothetical studies show that borophene is superior to any other material in terms of its ability to contain hydrogen, with up to 15% of its own mass.

Another possibility of borophene is catalysis, which separates hydrogen atoms into hydrogen ions, separating water into ions of hydrogen and oxygen. This makes borophene can be used to generate energy from water with greater efficiency than ever before.
It is the reaction of borophene that makes it easy to oxidize, which makes it difficult to experiment. Scientists need to study to overcome this weakness of it. However, they still cannot produce bulk borophene for testing and application. There is a lot of work to do before the makers of borophene become promising materials.
You should read it
- ★ Lithium-Sulfur batteries will help smartphones work longer
- ★ Decode the mystery of the Mpemba effect
- ★ Building the hair of the mysterious death of nine Soviet scientists
- ★ Want to Get Into 3D Printing? Here's How to Start
- ★ Scientists find the true cause of the giant animal extinction in Australia